Thermodynamics
There are three laws of thermodynamics. These laws and the science of thermodynamics originated in the early days of the industrial revolution. Engineers and physicists studied heat engines, any engine that uses heat energy to perform useful work, to try to improve their efficiency.
The laws of thermodynamics place fundamental limits on the efficiencies of heat engines. Good thermodynamic engineering can in principle produce heat engines that perform near these limits. However even the very best engineers cannot design engines that exceed these limits because it would violate fundamental laws of physics.
Conservation of Energy
This law of conservation of energy was not known when the French engineer, Sadi Carnot (1796 – 1832), did his pioneering analysis on the efficiency of heat engines.
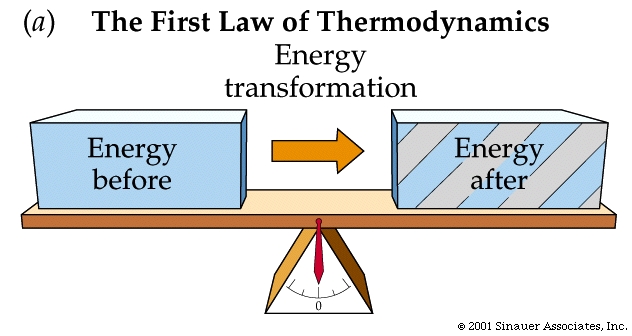
The First Law of Thermodynamics
The first law of thermodynamics relates the energy input for a heat engine, or other thermodynamic system, to the useful work output and the internal heat energy of the system. The total energy input equals the work output plus the change in internal heat energy of the system. Energy in = Work out + Change in internal heat energy. The change in the internal heat energy of the system is waste heat from the heat engine. That is why engines must be cooled.
If the internal heat energy does not change, then the energy going into a heat engine, or other system, equals the useful work output. In this case the efficiency of the engine is 100%. The first law of thermodynamics allows the possibility of an engine with 100% efficiency, but the second law of thermodynamics does not.
Conservation of Energy and the First Law
Heat is a form of energy. If the thermal energy of a system increases, then the random motions of individual atoms or molecules increase. This added heat energy and faster molecular motion increases the temperature.
It requires energy to do work. In physics a force must act over some distance to do work. The work, or the amount of energy required to do the work, is found by multiplying the force by the distance over which the force acts.
The energy going into a heat engine, the work the engine does, and the internal heat energy of the engine are all forms of energy. According to the law of conservation of energy the total energy must remain constant. That means the energy going into a system must equal the energy, or work, coming out plus the change in internal energy of the system. Hence the first law of thermodynamics, which states that the energy going into a heat engine must equal the useful work coming out plus the change in internal heat energy, is equivalent to the law of conservation of energy.
It is not possible to get something from nothing. No engine can do more useful work than the amount of energy input.